CLINICAL RESEARCH
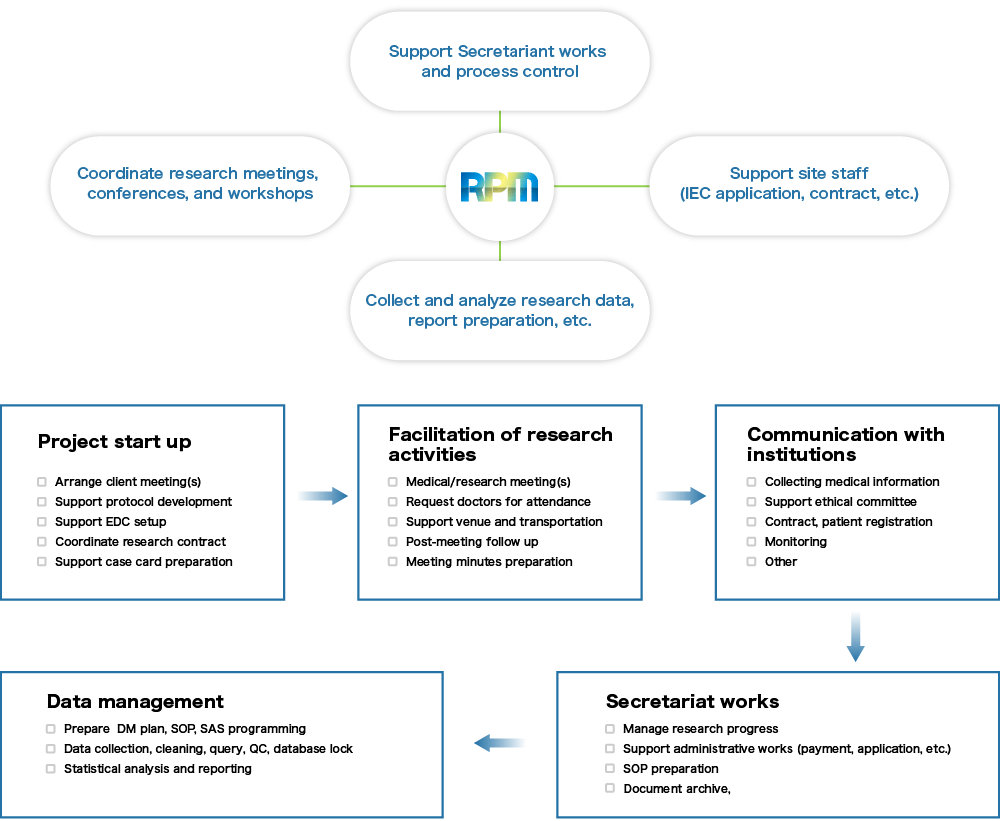
RPM provides a wide variety of assistance to medical institutions, academic organizations, and research professionals. Expected works can include the facilitation of networked functionalities (planning, ethical committee, data handling, monitoring, document archiving, coordination of stakeholders’ meetings, etc.) under the client-RPM contract. RPM considers the administrative coordination by the Secretariat Office to act as a hub, irrespective of disease area or academic field.
Clinical research work flow